

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:40, trinityanne1738
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 22:30, pookie879
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 23.06.2019 00:50, kaseywright3418
Which statement would indicate the presence of an acid
Answers: 3
Do you know the correct answer?
A sample of 2.45g aluminum oxide decomposes into 1.3g of aluminum and 1.15g of oxygen. What is the p...
Questions in other subjects:

English, 01.10.2019 16:30

Physics, 01.10.2019 16:30




Social Studies, 01.10.2019 16:30





 %
% %
%  %
% 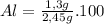 %
% %
% %
%




