
Chemistry, 06.03.2020 08:52, Nyasiabaltimore3
"To determine the amount of heroin in the mixture, you dissolve 1.00 g of the white powdery mixture in water in a 100.0-mL volumetric flask. You find that the solution has an osmotic pressure of 531 mm Hg at 25 °C. What is the composition of the mixture?"

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, hoytkeke6776
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 10:00, Cythina2007
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 19:00, hmontalvo22
How many moles are contained in 5.6 l of h2 at stp
Answers: 3

Chemistry, 23.06.2019 00:30, zaniathomasel
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1
Do you know the correct answer?
"To determine the amount of heroin in the mixture, you dissolve 1.00 g of the white powdery mixture...
Questions in other subjects:


Mathematics, 08.10.2019 21:30

Biology, 08.10.2019 21:30




Mathematics, 08.10.2019 21:30

Biology, 08.10.2019 21:30

Computers and Technology, 08.10.2019 21:30

Social Studies, 08.10.2019 21:30


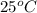
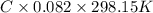
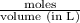 = 0.0285
= 0.0285
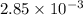
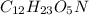 = x grams
= x grams
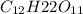 = (1.00 - x)
= (1.00 - x)
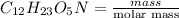
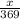
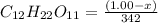
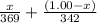
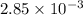
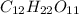 present is (1 - 0.346) g = 0.654 g.
present is (1 - 0.346) g = 0.654 g.



