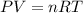Chemistry, 05.03.2020 20:07, nathanscastr02
In preparation for a demonstration, your professor brings a 1.50−L bottle of sulfur dioxide into the lecture hall before class to allow the gas to reach room temperature. If the pressure gauge reads 173 psi and the lecture hall is 20°C, how many moles of sulfur dioxide are in the bottle? In order to solve this problem, you will first need to calculate the pressure of the gas. Hint: The gauge reads zero when 14.7 psi of gas remains.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, BakerElsie02
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1



Chemistry, 23.06.2019 02:00, xoxoadara13ox07ck
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1
Do you know the correct answer?
In preparation for a demonstration, your professor brings a 1.50−L bottle of sulfur dioxide into the...
Questions in other subjects:





History, 12.04.2021 21:20


History, 12.04.2021 21:20

Mathematics, 12.04.2021 21:20

History, 12.04.2021 21:20