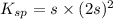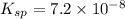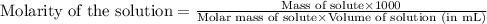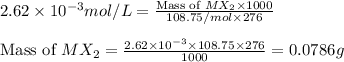Chemistry, 05.03.2020 15:09, ImmortalEnigmaYT
The solubility product constant for MX2 is 7.2 x 10-8. How many grams of MX2 (108.75 g/mol) will dissolve in 276 ml of water at 25°C. M is the metal and X is the anion. Enter as a number to 4 decimal places.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, annanikherrera
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3


Chemistry, 23.06.2019 01:00, birdman2540
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2

Chemistry, 23.06.2019 04:20, tyrickdavis1
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1
Do you know the correct answer?
The solubility product constant for MX2 is 7.2 x 10-8. How many grams of MX2 (108.75 g/mol) will dis...
Questions in other subjects:


Health, 23.04.2021 23:00



Mathematics, 23.04.2021 23:00

Mathematics, 23.04.2021 23:00

Mathematics, 23.04.2021 23:00

History, 23.04.2021 23:00



 that will dissolve is 0.0786 grams
that will dissolve is 0.0786 grams
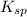 for above equation follows:
for above equation follows: