
Chemistry, 05.03.2020 10:11, trevorhenyan51
A certain mass of carbon reacts with 13.6 g of oxygen to form carbon monoxide. grams of oxygen would react with that same mass of carbon to form carbon dioxide, according to the law of multiple proportions.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, dayjionneam
In the analysis of hair and fiber samples, which does a compound comparison microscope allow for that a conventional compound microscope does not? a. simultaneous observation b. polarization c. fluorescence d. higher magnification
Answers: 2

Chemistry, 22.06.2019 07:30, 10040813
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 12:30, robert7248
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 20:30, allofthosefruit
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3
Do you know the correct answer?
A certain mass of carbon reacts with 13.6 g of oxygen to form carbon monoxide. grams of oxygen woul...
Questions in other subjects:

English, 03.02.2021 02:30

Spanish, 03.02.2021 02:30

Mathematics, 03.02.2021 02:30

Health, 03.02.2021 02:30

Arts, 03.02.2021 02:30



Mathematics, 03.02.2021 02:30


Chemistry, 03.02.2021 02:30


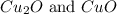
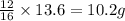 of carbon
of carbon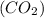 :
: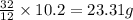 of oxygen
of oxygen



