
Chemistry, 05.03.2020 05:16, calmicaela12s
Consider the unbalanced equation for the combustion of hexane: C6H14 (g) O2 (g) --> CO2 (g) H2O (g) Balance the equation and determine how many moles of O2 are required to react completely with 7.2 moles of C6H14.

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 16:30, Kathryn014
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 22.06.2019 23:30, sanociahnoel
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
Do you know the correct answer?
Consider the unbalanced equation for the combustion of hexane: C6H14 (g) O2 (g) --> CO2 (g) H2O (...
Questions in other subjects:


Mathematics, 01.12.2020 03:20



Mathematics, 01.12.2020 03:20


Mathematics, 01.12.2020 03:20

English, 01.12.2020 03:20



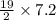 .
.



