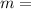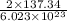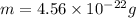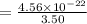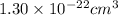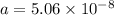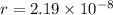Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, speris1443
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1


Chemistry, 22.06.2019 09:10, aleilyg2005
Select the correct answer from each drop-down menu. describe what happens to a carbon-11 atom when it undergoes positron emission. the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3
Do you know the correct answer?
The atoms in barium metal are arranged in a bodycentered cubic unit cell. Calculate the radius of a...
Questions in other subjects:

Mathematics, 04.04.2020 23:25


History, 04.04.2020 23:29





Business, 04.04.2020 23:30


English, 04.04.2020 23:30


 10^-8
10^-8