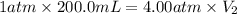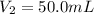Chemistry, 04.03.2020 23:45, DESI111609
4. The volume of a sample of a gas at STP is 200.0 ml. If the pressure is increased to 4.00 atmospheres (temperature constant), what is the new volume?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, ashleyjaslin
Calculate the expected ph values of the buffer systems from the experiments (a, b,c, d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2


Chemistry, 22.06.2019 12:00, sophiaa23
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 14:30, isaiahrodriguezsm17
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1
Do you know the correct answer?
4. The volume of a sample of a gas at STP is 200.0 ml. If the pressure is increased to 4.00 atmosphe...
Questions in other subjects:

Mathematics, 29.03.2021 19:00




Mathematics, 29.03.2021 19:00

Mathematics, 29.03.2021 19:00


History, 29.03.2021 19:00

English, 29.03.2021 19:00


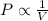
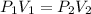
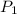 = initial pressure at STP = 1 atm
= initial pressure at STP = 1 atm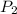 = final pressure = 4.00 atm
= final pressure = 4.00 atm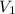 = initial volume at STP = 200.0 mL
= initial volume at STP = 200.0 mL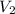 = final volume = ?
= final volume = ?