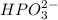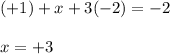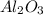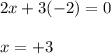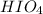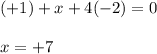Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, AleciaCassidy
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1


Chemistry, 22.06.2019 18:50, emily9656
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 23:00, NewKidnewlessons
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3
Do you know the correct answer?
Use the rules (in order) to assign oxidation numbers to each of the elements in the compounds below....
Questions in other subjects:

English, 16.02.2021 04:30



History, 16.02.2021 04:30





Spanish, 16.02.2021 04:30