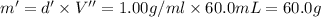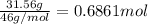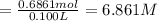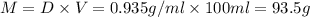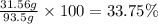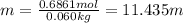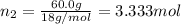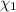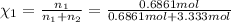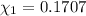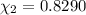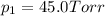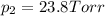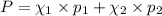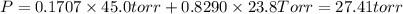Chemistry, 04.03.2020 02:22, bgallman153p71edg
Grey Goose ® vodka has an alcohol content of 40.0 % (v/v). Assuming that vodka is composed of only ethanol and water answer the following questions. Note: The molar masses of water and ethanol are 18.0 g and 46.0 g, respectively. The densities of water, ethanol, and this vodka mixture are 1.00 g/mL, 0.789 g/mL, and 0.935 g/mL, respectively
a. Calculate the molarity of ethanol in this vodka, assuming that water is the solvent.
b. Calculate the percent by mass of ethanol % (m/m) in this vodka.
c. Calculate the molality of ethanol in this vodka assuming that water is the solvent.
d. Calculate the mole fractions of ethanol and water in this vodka.
e. Calculate the vapor pressure, in torr, of this vodka at 25.0 oC if the vapor pressures of pure water and ethanol are 23.8 torr and 45.0 torr, respectively?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:10, andersonemma2222
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 12:30, masteroftheuniverse3
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 14:00, hannahhoskings6989
What was bohr’s contribution to the planetary model
Answers: 1
Do you know the correct answer?
Grey Goose ® vodka has an alcohol content of 40.0 % (v/v). Assuming that vodka is composed of only e...
Questions in other subjects:


Mathematics, 23.09.2019 11:30

History, 23.09.2019 11:30

Social Studies, 23.09.2019 11:30


History, 23.09.2019 11:30



Health, 23.09.2019 11:30

Mathematics, 23.09.2019 11:30