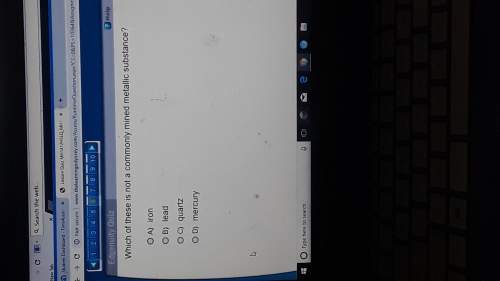
Chemistry, 03.03.2020 18:32, aleilyg2005
Methanol (CH3OH) is made by reacting gaseous carbon monoxide (CO) and gaseous hydrogen (H2).
A. What is the theoretical yield of methanol if you react 37.5 kg of CO(g) with 4.60 kg of H2(g)?
B. You found that 1.83 x 104 g of methanol is actually produced. What is the percent yield of methanol?

Answers: 2
Other questions on the subject: Chemistry



Do you know the correct answer?
Methanol (CH3OH) is made by reacting gaseous carbon monoxide (CO) and gaseous hydrogen (H2).
<...
<...
Questions in other subjects:


Mathematics, 06.05.2020 03:13

Medicine, 06.05.2020 03:13






Biology, 06.05.2020 03:13