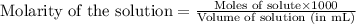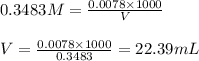Chemistry, 03.03.2020 05:59, egaitapierreval
Oxalic acid is a diprotic acid. If a solid material contains 53.66 percent of oxalic acid (H 2C 2O 4), by mass, then a 0.6543-g sample of that solid will require mL of 0.3483 M NaOH for neutralization. 11.19 97.78 28.59 1.119 22.39

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, tchase0616
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 12:30, kingbot350
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1


Chemistry, 22.06.2019 21:30, sierradanielle9280
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3
Do you know the correct answer?
Oxalic acid is a diprotic acid. If a solid material contains 53.66 percent of oxalic acid (H 2C 2O 4...
Questions in other subjects:




Physics, 06.03.2022 17:20



English, 06.03.2022 17:30


Physics, 06.03.2022 17:30

Geography, 06.03.2022 17:30


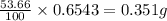
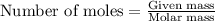
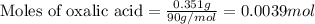
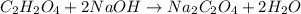
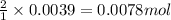 of NaOH
of NaOH