
A buffer solution that is 0.100 M in both HCOOH and HCOOK has a pH = 3.75. A student says that if a very small amount of 0.100 M HCl is added to the buffer, the pH will decrease by a very small amount. Which of the following best supports the student's claim? (A) HCOO will accept a proton from HCl to produce more HCOOH and H2O(B) HCOOH will accept a proton from HCl to produce more HCOO and H2O. (C) HCOO wil donate a proton to HCl to produce more HCOOH and H2O (D) HCOOH will donate a proton to HCl to produce more HCOO and H2O.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, heavyhearttim
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 17:40, aguilarjose
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 22:00, choatefarmsus
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3
Do you know the correct answer?
A buffer solution that is 0.100 M in both HCOOH and HCOOK has a pH = 3.75. A student says that if a...
Questions in other subjects:


History, 29.01.2020 02:13



Physics, 29.01.2020 02:13

Chemistry, 29.01.2020 02:13

Chemistry, 29.01.2020 02:13

Mathematics, 29.01.2020 02:13



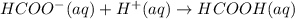
![pk_{a} + log \frac{[HCOO^{-}]}{[HCOOH]}](/tpl/images/0531/9252/8a009.png)
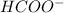 is directly proportional to pH. Hence, when there occurs a decrease in the pH of the solution the
is directly proportional to pH. Hence, when there occurs a decrease in the pH of the solution the ![[HCOO^{-}]](/tpl/images/0531/9252/bee9e.png) will also decrease.
will also decrease. 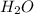 , best supports the student's claim.
, best supports the student's claim.




