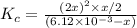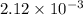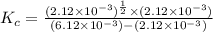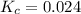Chemistry, 03.03.2020 06:12, abigaleschwartz
A 100 L reaction container is charged with 0.612 mol of NOBr, which decomposes at a certain temperature** (say between 100 and 150 oC) according to the following reaction:
NOBr(g) ? NO(g) + 0.5Br2(g)
At equilibrium the bromine concentration is 2.12x10-3 M. Calculate Kc (in M0.5)
**Not specifying the temperature allows for a more liberal use of random numbers.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:50, Mercedes12152002
Where are chemicals found at work? a. only in cleaning products b. only in carpets and paint c. in every area of work d. only in food preparation submit
Answers: 1

Chemistry, 22.06.2019 07:30, reaperqueen21
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 11:20, Jessicadiaz8602
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 18:10, sangamlama
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
Do you know the correct answer?
A 100 L reaction container is charged with 0.612 mol of NOBr, which decomposes at a certain temperat...
Questions in other subjects:






Business, 03.07.2021 17:10


Mathematics, 03.07.2021 17:10



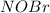 = 0.612 mole
= 0.612 mole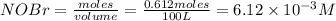
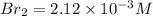
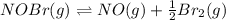
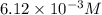 0 0
0 0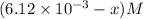 x x/2
x x/2 ![K_c=\frac{[NO]^2[Br_2]}{[NOBr]}](/tpl/images/0532/0141/32da8.png)