

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:30, sydneip6174
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 23.06.2019 01:10, dontcareanyonemo
Can someone check my work 98 5.05 acids and bases for this assignment you will be comparing acids and bases. the chart below will you organize the information needed: acids bases chemical properties (2) deodorant detergent vinger dish soap physical properties (2) orange juice toilet cleaner drain cleaner window cleaner ph level acid ph goes from 0-4 bases ph goes from 10-14 examples around you (2) vinger coffee lemon juice dark chocolate
Answers: 3

Chemistry, 23.06.2019 09:00, hunterwilliams375
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate according to the following equation:
Answers: 2

Chemistry, 23.06.2019 09:50, jay4881
T(s) in2os] (m) 0 185 2.39 546 1.90 725 1.70 the decomposition of n205 can be described by the equation 2.68 given these data for the reaction at 45°c in carbon tetrachloride solution, calculate the average rate of reaction for each successive time interval. ntr s to 185 s 185 s to 546 s 546 s to 725 s number number number reaction rate: m/s m/s m/s
Answers: 1
Do you know the correct answer?
Assume that five weak acids, identified only by numbers (1, 2, 3, 4, and 5) have the following ioniz...
Questions in other subjects:


Mathematics, 26.05.2020 10:57

Mathematics, 26.05.2020 10:57

History, 26.05.2020 10:57

Mathematics, 26.05.2020 10:57

Biology, 26.05.2020 10:57

Mathematics, 26.05.2020 10:57


Physics, 26.05.2020 10:57


![K_a = \frac{[H^+]^2}{[HA]}](/tpl/images/0531/8279/683f8.png)
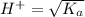 pH = -Log{H⁺]
pH = -Log{H⁺]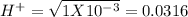 , pH = 1.5
, pH = 1.5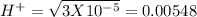 , pH = 2
, pH = 2 pH = 3
pH = 3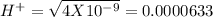 pH = 4
pH = 4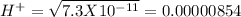 pH = 5
pH = 5 



