Chemistry, 03.03.2020 04:53, GhostElite6383
A 0.9679-g sample containing dimethylphthalate, (194.19 g/mol), and unreactive species was refluxed with 50.00 mL of 0.1215 M to hydrolyze the ester groups (this process is called saponification).
C6H4(COOCH3)2 + 2OH>> C6H4(COO)-2 + H2O
After the reaction was complete, the excess NaOH was back titrated with 32.25mL of 0.1251M HCl. Calculate the percentage of dimethylphthalate in the sample.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, tbiles99
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1


Chemistry, 22.06.2019 16:50, briansalazar17
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3
Do you know the correct answer?
A 0.9679-g sample containing dimethylphthalate, (194.19 g/mol), and unreactive species was refluxed...
Questions in other subjects:



Geography, 02.07.2019 01:00

Health, 02.07.2019 01:00







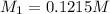


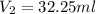
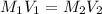
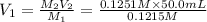
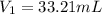
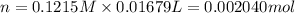

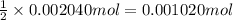 of dimethylphthalate
of dimethylphthalate