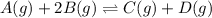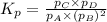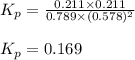Answers: 2
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 05:00, lifeoflashay1659
He nucleus contains the cells genetic material in the form of dna. dna is organized into our chromosomes, which are made up of thousands of that determine our traits.
Answers: 1

Chemistry, 23.06.2019 07:30, fernandancon1872
Assume that 13.5 g solid aluminum (al) react with hcl to produce solid aluminum chloride (alcl3) salt and gaseous hydrogen (h2) at standard temperature and pressure.
Answers: 1

Chemistry, 23.06.2019 11:30, elizebeth4501
Which of these have the same number of particles as 1 mole of water h2o
Answers: 1

Chemistry, 23.06.2019 17:00, jayjayanyway04
During which of the following phases of the moon do we see the left half of the moon as lit? full moon first quarter moon gibbous moon third quarter moon any is greatly : )
Answers: 1
Do you know the correct answer?
A(g) + 2B(g) → C(g) + D(g)If you initially start with 1.00 atm of both A and B and find that at equi...
Questions in other subjects:







Mathematics, 28.05.2020 18:58




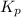 for the reaction is 0.169
for the reaction is 0.169