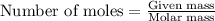Chemistry, 03.03.2020 02:52, wedestttefera
Consider the following chemical reaction: 2H2O(l)→2H2(g)+O2(g) What mass of H2O is required to form 1.3 L of O2 at a temperature of 295 K and a pressure of 0.926 atm ?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, salvadorperez26
Asolution has a ca2+ concentration of 0.049 m and an f- concentration is 0.147 m at equilibrium. the value of ksp for caf2 at 25°c is 4.0 x 10-11. will this solution form a precipitate? yes no
Answers: 3


Chemistry, 22.06.2019 03:30, fbillinton
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 05:30, sethjohnson386pbnm3x
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3
Do you know the correct answer?
Consider the following chemical reaction: 2H2O(l)→2H2(g)+O2(g) What mass of H2O is required to form...
Questions in other subjects:

Mathematics, 15.06.2021 18:10


History, 15.06.2021 18:10

Mathematics, 15.06.2021 18:10





Mathematics, 15.06.2021 18:10


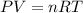
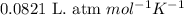
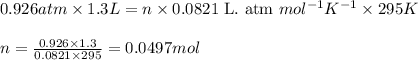
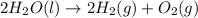
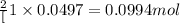 of water
of water