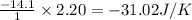Chemistry, 03.03.2020 01:25, KenzieD7876
H2(g) + F2(g)2HF(g) Using standard thermodynamic data at 298K, calculate the entropy change for the surroundings when 2.20 moles of H2(g) react at standard conditions. S°surroundings = J/K

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:10, josephpezza18
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 09:40, cheesecake1919
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3
Do you know the correct answer?
H2(g) + F2(g)2HF(g) Using standard thermodynamic data at 298K, calculate the entropy change for the...
Questions in other subjects:






Mathematics, 05.05.2020 02:41




Mathematics, 05.05.2020 02:41


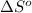 for the surrounding when given amount of hydrogen gas is reacted is -31.02 J/K
for the surrounding when given amount of hydrogen gas is reacted is -31.02 J/K![\Delta S^o_{rxn}=\sum [n\times \Delta S^o_{(product)}]-\sum [n\times \Delta S^o_{(reactant)}]](/tpl/images/0531/3518/52737.png)
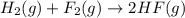
![\Delta S^o_{rxn}=[(2\times \Delta S^o_{(HF(g))})]-[(1\times \Delta S^o_{(H_2(g))})+(1\times \Delta S^o_{(F_2(g))})]](/tpl/images/0531/3518/0a21f.png)
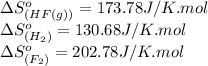
![\Delta S^o_{rxn}=[(2\times (173.78))]-[(1\times (130.68))+(1\times (202.78))]\\\\\Delta S^o_{rxn}=14.1J/K](/tpl/images/0531/3518/7b4e1.png)