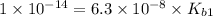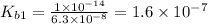Chemistry, 03.03.2020 01:16, jameanch7182
Let us write the appropriate equilibria and associate the correction values. Remember, we will want to calculate the concentrations of all species in a 0.390 M Na ₂SO₃ (sodium sulfite) solution. The ionization constants for sulfurous acid are = 1.4 × 10⁻² and = 6.3 × 10⁻⁸.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, cutebab4786
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles, has been added? 0.308 mol fecl3 initially present
Answers: 1



Chemistry, 23.06.2019 00:10, graceception
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3
Do you know the correct answer?
Let us write the appropriate equilibria and associate the correction values. Remember, we will want...
Questions in other subjects:

Social Studies, 18.08.2021 08:10





English, 18.08.2021 08:10


Mathematics, 18.08.2021 08:10

English, 18.08.2021 08:10

Mathematics, 18.08.2021 08:10


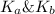 is given by :
is given by :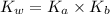
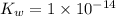 = Ionic prodcut of water
= Ionic prodcut of water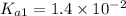
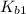 :
: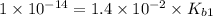
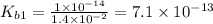
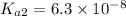
 :
: