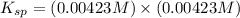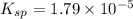Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, aschool2000
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 21:00, rah45
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1

Chemistry, 22.06.2019 21:30, djdjdjdbdbjx
What is another way to determine mass times acceleration?
Answers: 1
Do you know the correct answer?
At 25 °C, an aqueous solution has an equilibrium concentration of 0.00423 0.00423 M for a generic ca...
Questions in other subjects:


Mathematics, 16.04.2021 21:00


Mathematics, 16.04.2021 21:00

Mathematics, 16.04.2021 21:00


Mathematics, 16.04.2021 21:00


English, 16.04.2021 21:00


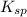 of the generic salt AB is,
of the generic salt AB is, 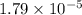
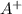 = 0.00423 M
= 0.00423 M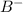 = 0.00423 M
= 0.00423 M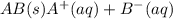
![K_{sp}=[A^+][B^-]](/tpl/images/0531/1161/7fe85.png)