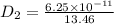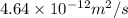Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, goodygoodgirlygirl
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 11:50, tajanaewilliams77
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 21:30, Turtlelover05
How can the periodic table be used to predict the behavior of elements?
Answers: 1

Chemistry, 23.06.2019 00:00, PlzNoToxicBan
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1
Do you know the correct answer?
The activation energy for the diffusion of carbon in chromium is 111,000 J/mol. Calculate the diffus...
Questions in other subjects:


History, 23.11.2019 15:31




Mathematics, 23.11.2019 15:31



Biology, 23.11.2019 15:31


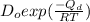
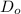 = constant
= constant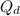 = activation energy
= activation energy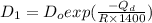
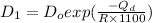
![\frac{D_{1}}{D_{2}} = exp[\frac{-Q_{d}}{1400 R} + \frac{Q}{1100 R}]](/tpl/images/0530/9405/91326.png)
![\frac{6.25 \times 10^{-11}}{D_{2}} = exp [\frac{-Q_{d}}{R}(\frac{-300}{1400 \times 1100})]](/tpl/images/0530/9405/0c339.png)
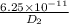 = exp (2.6)
= exp (2.6)