
Chemistry, 02.03.2020 21:07, jahnoibenjamin
Which of the following solutions would have the highest [H3O+]? Assume that they are all 0.10 M in acid at 25∘C. The acid is followed by its Ka value. C) HNO2, 4.6 × 10-4 B) HCN, 4.9 × 10-10 E) HClO2, 1.1 × 10-2 A) HF, 3.5 × 10-4 D) HCHO2, 1.8 × 10-4

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:10, josephpezza18
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 04:00, dustinsampsin2486
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 10:00, alexabdercmur
Why is the structure of molecule important to its function?
Answers: 1
Do you know the correct answer?
Which of the following solutions would have the highest [H3O+]? Assume that they are all 0.10 M in a...
Questions in other subjects:

Mathematics, 12.12.2020 16:00


Mathematics, 12.12.2020 16:00

Chemistry, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00

History, 12.12.2020 16:00


Mathematics, 12.12.2020 16:00



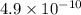
![[H^+]=\sqrt{(K_aC)}](/tpl/images/0530/6657/2da9a.png)
![pH=-\log [H^+]](/tpl/images/0530/6657/37e81.png)
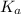 (dissociation constant) increases then the value of hydrogen ion concentration also increases and the value of pH decreases.
(dissociation constant) increases then the value of hydrogen ion concentration also increases and the value of pH decreases.



