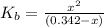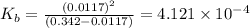Chemistry, 02.03.2020 20:38, mrmendrala
N the laboratory, a general chemistry student measured the pH of a 0.342 M aqueous solution of ethylamine, C2H5NH2 to be 12.067. Use the information she obtained to determine the Kb for this base.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:50, donttrip10
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state. a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2

Chemistry, 22.06.2019 22:10, steven0448
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

Chemistry, 23.06.2019 02:00, matthewsorrow02
What is the mass of 0.750 mole of aluminum oxide, al2o3?
Answers: 1
Do you know the correct answer?
N the laboratory, a general chemistry student measured the pH of a 0.342 M aqueous solution of ethyl...
Questions in other subjects:

Mathematics, 23.12.2019 22:31


Biology, 23.12.2019 22:31

Mathematics, 23.12.2019 22:31


Chemistry, 23.12.2019 22:31

Mathematics, 23.12.2019 22:31


History, 23.12.2019 22:31


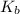 of the an ethylamine is
of the an ethylamine is  .
.![pOH=-\log[OH^-]](/tpl/images/0530/5572/fe336.png)
![1.933=-\log[OH^-]](/tpl/images/0530/5572/b301a.png)
![[OH^-]=0.0117 M](/tpl/images/0530/5572/660e1.png)

![K_b=\frac{[C_2H_5NH_3^{+}][OH^-]}{[C_2H_5NH_2]}](/tpl/images/0530/5572/63c9d.png)