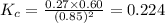Chemistry, 02.03.2020 21:19, braxtengames
Calculate Kc for the reaction: 2 HI(g) ⇄ H2(g) + I2(g) given that the concentrations of each species at equilibrium are as follows: [HI] = 0.85 mol/L, [I2] = 0.60 mol/L, [H2] = 0.27 mol/L.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:50, deanlmartin
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1


Chemistry, 21.06.2019 22:40, wiltseliz4800
What does the process of natural selection involve
Answers: 1
Do you know the correct answer?
Calculate Kc for the reaction: 2 HI(g) ⇄ H2(g) + I2(g) given that the concentrations of each species...
Questions in other subjects:


Biology, 05.10.2019 19:00


Mathematics, 05.10.2019 19:00

History, 05.10.2019 19:00


History, 05.10.2019 19:00

Mathematics, 05.10.2019 19:00



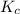 for the given reaction is 0.224
for the given reaction is 0.224 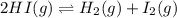
![K_c=\frac{[H_2][I_2]}{[HI]^2}](/tpl/images/0530/7257/ef85e.png)
![[HI]_{eq}=0.85M](/tpl/images/0530/7257/9952a.png)
![[H_2]_{eq}=0.27M](/tpl/images/0530/7257/01e4f.png)
![[I_2]_{eq}=0.60M](/tpl/images/0530/7257/de9f5.png)