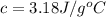Chemistry, 02.03.2020 19:22, PONBallfordM89
A 11.6 g piece of metal is heated to 98°C and dropped into a calorimeter containing 50.0 g of water (specific heat capacity of water is 4.18 J/g°C) initially at 20.5°C. The empty calorimeter has a heat capacity of 125 J/K. The final temperature of the water is 28.2°C. Ignoring significant figures, calculate the specific heat of the metal.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, ronny80
Supongamos que estás estudiando dos estrellas. ambas estrellas tienen la misma magnitud aparente, pero la estrella a tiene una magnitud absoluta mayor que la estrella b. ¿que puedes decir acerca de la distancia a la tierra de estas dos estrellas?
Answers: 3

Chemistry, 21.06.2019 19:30, aedmund1225
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1


Chemistry, 22.06.2019 06:20, Naysa150724
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3
Do you know the correct answer?
A 11.6 g piece of metal is heated to 98°C and dropped into a calorimeter containing 50.0 g of water...
Questions in other subjects:




Mathematics, 16.07.2019 10:30



Mathematics, 16.07.2019 10:30




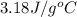
![q=-[q_1+q_2]](/tpl/images/0530/4537/f9283.png)
![m\times c\times (T_f-T_1)=-[c_1\times (T_f-T_2)+m_2\times c_2\times (T_f-T_2)]](/tpl/images/0530/4537/372b8.png)
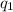 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter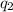 = heat absorbed by the water
= heat absorbed by the water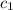 = specific heat of calorimeter =
= specific heat of calorimeter = 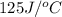
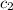 = specific heat of water =
= specific heat of water = 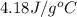
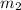 = mass of water = 50.0 g
= mass of water = 50.0 g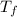 = final temperature =
= final temperature = 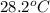
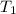 = temperature of metal =
= temperature of metal = 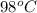
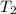 = temperature of water =
= temperature of water = 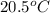
![11.6g\times c\times (28.2-98)^oC=-[125J/^oC\times (28.2-20.5)^oC+50.0g\times 4.18J/g^oC\times (28.2-20.5)^oC]](/tpl/images/0530/4537/17b20.png)