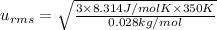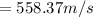Chemistry, 02.03.2020 19:29, firenation18
A 0.50 m3 gas tank holds 3.0 moles of ideal diatomic nitrogen gas at a temperature of 350 K. The atomic mass of nitrogen is 14 g/mol. What is the rms speed of the molecules? (The Boltzmann constant is 1.38 × 10-23 J/K, NA = 6.022 × 1023 molecules/mol.)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 12:10, kaitlynbernatz2778
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 13:00, aleilyg2005
If two objects at different te, peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 17:30, mwest200316
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1
Do you know the correct answer?
A 0.50 m3 gas tank holds 3.0 moles of ideal diatomic nitrogen gas at a temperature of 350 K. The ato...
Questions in other subjects:




Biology, 01.04.2020 22:02


Mathematics, 01.04.2020 22:02

Biology, 01.04.2020 22:02

Mathematics, 01.04.2020 22:02

Mathematics, 01.04.2020 22:02


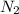 , M= 2 × 14 g/mol = 28 g/mol
, M= 2 × 14 g/mol = 28 g/mol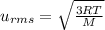
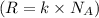
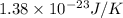
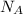 = Avogadro number
= Avogadro number