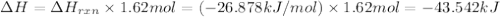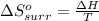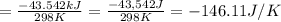Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:40, georgehall3027
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 06:10, Kianna000
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1

Chemistry, 22.06.2019 10:00, melissa9882
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 15:30, dylannhandy
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2
Do you know the correct answer?
I2(g) + Cl2(g)2ICl(g) Using standard thermodynamic data at 298K, calculate the entropy change for th...
Questions in other subjects:


Mathematics, 05.05.2020 23:37

History, 05.05.2020 23:37






Computers and Technology, 05.05.2020 23:37

Mathematics, 05.05.2020 23:37


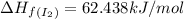
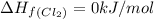
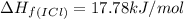
![\Delta H_{rxn}=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0530/3455/db29b.png)
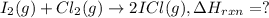
![\Delta H_{rxn}=[(2\times \Delta H_f_{(ICl)})]-[(1\times \Delta H_f_{(I_2)})+(1\times \Delta H_f_{(Cl_2)})]](/tpl/images/0530/3455/4a661.png)
![=[2\times 17.78 kJ/mol]-[1\times 0 kJ/mol+1\times 62.436 kJ/mol]=-26.878 kJ/mol](/tpl/images/0530/3455/6a4a8.png)