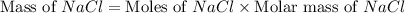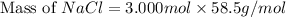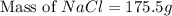Chemistry, 02.03.2020 18:22, squawk1738
The molality equation also requires the moles of solute. The molarity describes 3.000 moles of NaCl per 1 L of solution. Use the moles of NaCl to solve for the mass of NaCl. Note: use the molar mass of NaCl rounded to 4 significant figures.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, dinosaur10
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 15:30, elizabethprasad2
The reactions of photosynthesis occur in the of plant cell? a. mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 20:00, bettybales1986
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2
Do you know the correct answer?
The molality equation also requires the moles of solute. The molarity describes 3.000 moles of NaCl...
Questions in other subjects:


History, 31.08.2019 19:20



English, 31.08.2019 19:20

Physics, 31.08.2019 19:20

Mathematics, 31.08.2019 19:20


Law, 31.08.2019 19:20

English, 31.08.2019 19:20


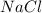 = 3.000 mol
= 3.000 mol