

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, kkruvc
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 21.06.2019 23:00, slugmilk1090
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 16:00, winnie45
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 21:30, Turtlelover05
How can the periodic table be used to predict the behavior of elements?
Answers: 1
Do you know the correct answer?
Calculate the pH and fraction of dissociation ( α ) for each of the acetic acid ( CH 3 COOH , p K a...
Questions in other subjects:

Mathematics, 28.12.2020 17:00


Business, 28.12.2020 17:00


Social Studies, 28.12.2020 17:00



English, 28.12.2020 17:00



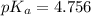
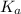
![pK_a=-\log[K_a]](/tpl/images/0530/3712/78bbf.png)
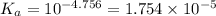
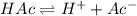
![K_a=\frac{[H^+][Ac^-]}{[HAc]}](/tpl/images/0530/3712/ce0d8.png)
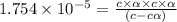
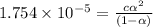
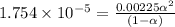
![[H^+]=c\alpha = 0.00225M\times 0.08448=0.0001901 M](/tpl/images/0530/3712/81253.png)
![pH=-\log[H^+]](/tpl/images/0530/3712/cf945.png)
![=-\log[0.0001901 M]=3.72](/tpl/images/0530/3712/5ff4a.png)




