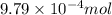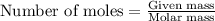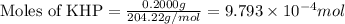Chemistry, 02.03.2020 17:28, janreyes39
As part of Lab 4 you will make and standardize a solution of NaOH(aq). Suppose in the lab you measure the solid NaOH and dissolve it into 100.0 mL of water. You then measure 0.2000 g of KHP (204.22 g/mol) and place it in a clean, dry 100-mL beaker, and then dissolve the KHP in about 25 mL of water and add a couple of drops of phenolphthalein indicator. You titrate this with your NaOH(aq) solution and find that the titration requires 9.78 mL of NaOH(aq). Part 1: How many moles of KHP are in your sample

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 17:30, kaytonleeb
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 21:30, sarah192002
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1

Chemistry, 23.06.2019 04:00, ayoismeisjjjjuan
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2
Do you know the correct answer?
As part of Lab 4 you will make and standardize a solution of NaOH(aq). Suppose in the lab you measur...
Questions in other subjects:





History, 06.12.2019 18:31

History, 06.12.2019 18:31

Spanish, 06.12.2019 18:31