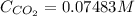Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, Arealbot
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 22.06.2019 05:50, aylengarcia090
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 17:30, nijanicole164
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2
Do you know the correct answer?
The Henry's law constant for CO 2 ( g ) CO2(g) in water at 25 ∘ C 25 ∘C is 29.4 bar⋅M − 1 29.4 bar·M...
Questions in other subjects:


Chemistry, 08.04.2020 22:00


Mathematics, 08.04.2020 22:00

Chemistry, 08.04.2020 22:00

English, 08.04.2020 22:00





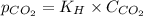
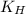 = Henry's constant =
= Henry's constant = 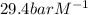
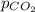 = partial pressure of carbonated drink = 2.20 bar
= partial pressure of carbonated drink = 2.20 bar