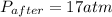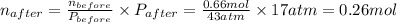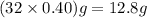A rigid tank contains 0.66 mol of oxygen (O2). Find the mass of oxygen that must be withdrawn from the tank to lower the pressure of the gas from 43 atm to 17 atm. Assume that the volume of the tank and the temperature of the oxygen are constant during this operation. Answer in units of g.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, justinerodriguz2878
What are the major types of a chemical compound
Answers: 2

Chemistry, 22.06.2019 16:50, shaylawaldo11
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 17:00, destinyycooper
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
Do you know the correct answer?
A rigid tank contains 0.66 mol of oxygen (O2). Find the mass of oxygen that must be withdrawn from t...
Questions in other subjects:

Mathematics, 12.02.2021 22:10



Social Studies, 12.02.2021 22:10




Mathematics, 12.02.2021 22:10

Mathematics, 12.02.2021 22:10

Mathematics, 12.02.2021 22:10


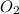 must be withdrawn from tank
must be withdrawn from tank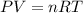
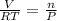
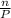 ratio will also be constant before and after removal of
ratio will also be constant before and after removal of 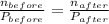
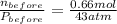 and
and