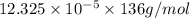Chemistry, 02.03.2020 17:10, ozzypurple05
Calculate the solubility (in g/L) of CaSO 4 ( s ) CaSO4(s) in 0.400 M Na 2 SO 4 ( aq ) at 25 ° C 0.400 M Na2SO4(aq) at 25°C. The K sp Ksp of CaSO 4 CaSO4 is 4.93 × 10 − 5 4.93×10−5.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 12:10, yootmytoot
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution. calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 12:40, jaylen2559
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1
Do you know the correct answer?
Calculate the solubility (in g/L) of CaSO 4 ( s ) CaSO4(s) in 0.400 M Na 2 SO 4 ( aq ) at 25 ° C 0.4...
Questions in other subjects:

Mathematics, 12.10.2019 10:00

English, 12.10.2019 10:00

Mathematics, 12.10.2019 10:00

Chemistry, 12.10.2019 10:00


English, 12.10.2019 10:10


Mathematics, 12.10.2019 10:10



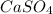 is as follows.
is as follows.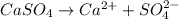
![K_{sp} = [Ca^{2+}][SO^{2-}_{4}]](/tpl/images/0530/1345/0d847.png)
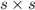
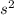
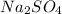 is given as 0.4 M.
is given as 0.4 M. ![[Na_{2}SO_{4}] = [SO^{2-}_{4}]](/tpl/images/0530/1345/ebfe8.png) = 0.4 M
= 0.4 M![4.93 \times 10^{-5} = [Ca^{2+}] \times 0.4](/tpl/images/0530/1345/e5147.png)
![[Ca^{2+}]](/tpl/images/0530/1345/17576.png) = 12.325 \times 10^{-5}[/tex]
= 12.325 \times 10^{-5}[/tex]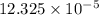 .
.