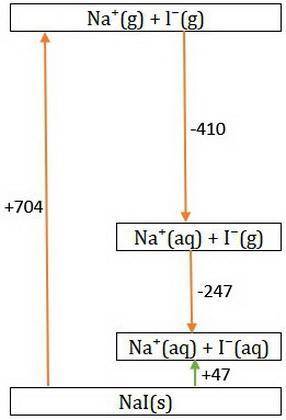
Consider Nal → Na+ + - and the following information.
Hlat = -704 kJ/mol
AHhydr of Na+=...

Chemistry, 01.03.2020 00:17, 2022maldonadoleonel
Consider Nal → Na+ + - and the following information.
Hlat = -704 kJ/mol
AHhydr of Na+= -410.0 kJ/mol
AHhydr of -= -247 kJ/mol
What is the AHSol of this compound? Use AHsol = -AHlat + AHhydr.
0-867 kJ/mol
|-867.0 kJ/mol
0 47 kJ/mol
0 47.0 kJ/mol

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 13:30, kassandrarosario1115
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 19:00, cindyroxana229
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 19:00, nayashuntel
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1
Do you know the correct answer?
Questions in other subjects:

English, 17.03.2020 15:58

Mathematics, 17.03.2020 15:59


Mathematics, 17.03.2020 15:59

Mathematics, 17.03.2020 16:00




Mathematics, 17.03.2020 16:01