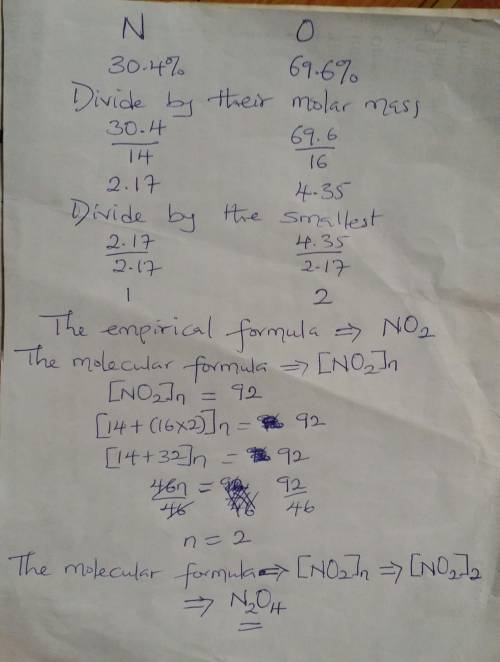Extension Questions:
17. An oxide of nitrogen is found to contain 69.6% oxygen and has a molar...

Chemistry, 29.02.2020 07:40, isabelperez063
Extension Questions:
17. An oxide of nitrogen is found to contain 69.6% oxygen and has a molar mass of 92.0 g/mole.
a. What is the % nitrogen in this compound?
b. Find the empirical formula and molecular formula for this compound.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, ciarakelly636owuiup
Asample of the male sex hormone testosterone, c19h28o2, contains 3.88×10^21 atoms of hydrogen.(a) how many atoms of carbon does it contain? (b) how many molecules of testosterone does it contain? (c) how many moles of testosterone does it contain? (d) what is the mass of this sample in grams?
Answers: 1

Chemistry, 22.06.2019 06:40, alyons60
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Do you know the correct answer?
Questions in other subjects:



Computers and Technology, 22.04.2021 22:40



Mathematics, 22.04.2021 22:40