
Chemistry, 29.02.2020 04:26, sierra6816
A flask is filled with 6.0 atm of N2 and 6.0 atm of H2. The gases react and NH3 is formed. What is the pressure in the flask after the reaction occurs as completely as possible

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:20, Jessicadiaz8602
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1


Chemistry, 22.06.2019 17:00, brownvester44
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 20:00, kalcius9698
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
Do you know the correct answer?
A flask is filled with 6.0 atm of N2 and 6.0 atm of H2. The gases react and NH3 is formed. What is t...
Questions in other subjects:



Mathematics, 13.12.2021 18:30



Business, 13.12.2021 18:30

History, 13.12.2021 18:30

English, 13.12.2021 18:30

Mathematics, 13.12.2021 18:30


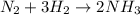
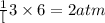 of nitrogen gas
of nitrogen gas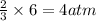 of ammonia
of ammonia



