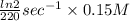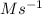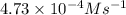Chemistry, 29.02.2020 03:23, tobywaffle1234
A series of experiments investigating the reaction of (CH3)3CCl with H2O to create (CH3)3OH produces a plot of Ln[(CH3)3CCl] vs. time that is linear with a negative slope. Suppose the reaction is carried out under conditions such that the half-life of the reaction is 2.20 x 102 s. What is the instantaneous rate of reaction when [(CH3)3CCl] = 0.15 M?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, sillslola816oxb5h7
An aqueous solution of hydroiodic acid is standardized by titration with a 0.186 m solution of calcium hydroxide. if 26.5 ml of base are required to neutralize 20.3 ml of the acid, what is the molarity of the hydroiodic acid solution? m hydroiodic acid
Answers: 1


Chemistry, 22.06.2019 02:00, lwattsstudent
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 12:00, carvajalj2520
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1
Do you know the correct answer?
A series of experiments investigating the reaction of (CH3)3CCl with H2O to create (CH3)3OH produces...
Questions in other subjects:


Mathematics, 04.12.2020 21:40

Mathematics, 04.12.2020 21:40

Mathematics, 04.12.2020 21:40

Mathematics, 04.12.2020 21:40

Mathematics, 04.12.2020 21:40




English, 04.12.2020 21:40


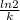
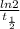
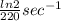
![[(CH_{3})_{3}CCl]^{1}](/tpl/images/0528/9812/8a6e5.png)