
Below are the reduction half reactions for chemolithoautotrophic nitrification, where ammonia is a source of electrons and energy and oxygen is the terminal electron acceptor. NO2- 6e- -> NH4 (-0.41 volts) O2 4e- -> 2H2O ( 0.82 volts) If you balance and combine the reactions so that 28 molecules of NH4 are oxidized to NO2-, how many molecules of O2 will be reduced to H2O

Answers: 2
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 21:30, djdjdjdbdbjx
What is another way to determine mass times acceleration?
Answers: 1
Do you know the correct answer?
Below are the reduction half reactions for chemolithoautotrophic nitrification, where ammonia is a s...
Questions in other subjects:


Mathematics, 27.09.2019 15:30




Biology, 27.09.2019 15:30

Mathematics, 27.09.2019 15:30


Biology, 27.09.2019 15:30


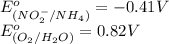
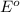 potential will always get reduced and will undergo reduction reaction. Here, oxygen will undergo reduction reaction will get reduced.
potential will always get reduced and will undergo reduction reaction. Here, oxygen will undergo reduction reaction will get reduced.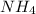 will undergo oxidation reaction and will get oxidized.
will undergo oxidation reaction and will get oxidized.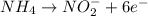 ( × 4)
( × 4)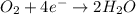 ( × 6)
( × 6)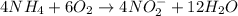
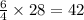 molecules of oxygen gas
molecules of oxygen gas



