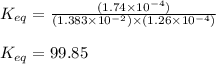Chemistry, 29.02.2020 02:52, eriksprincess13
An aqueous solution was prepared at 21 oC by mixing 7.00 mL 2.00 x 10-2mol L-1Fe3+, 2.00 mL 1.50 x 10-3 mol L-1SCN−, and 1.00 mL water. At equilibrium, the concentration of the product complex, [Fe(SCN)2+]eq was determined to be 1.74 x 10-4mol L-1. What is the value of the equilibrium constant K for the reaction of interest at 21 oC?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:40, markipler01
What is the study of how matter and energy interact? a. biology b. physics c. planetary science d. chemistry
Answers: 1

Chemistry, 22.06.2019 02:10, vapelordcarl69
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3


Chemistry, 22.06.2019 10:30, ciel8809
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1
Do you know the correct answer?
An aqueous solution was prepared at 21 oC by mixing 7.00 mL 2.00 x 10-2mol L-1Fe3+, 2.00 mL 1.50 x 1...
Questions in other subjects:

Social Studies, 02.04.2020 19:28


History, 02.04.2020 19:28

History, 02.04.2020 19:28

Mathematics, 02.04.2020 19:28


English, 02.04.2020 19:28


Mathematics, 02.04.2020 19:28

History, 02.04.2020 19:28


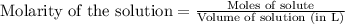 .....(1)
.....(1)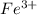 ions:
ions: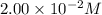
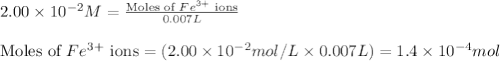
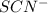 ions:
ions: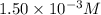
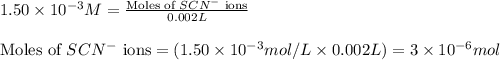
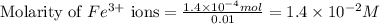
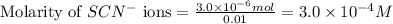
![[FeSCN^{2+}]](/tpl/images/0528/9422/797d4.png) complex follows:
complex follows:![Fe^{2+}+SCN^-\rightleftharpoons [FeSCN^{2+}]](/tpl/images/0528/9422/8a467.png)
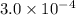
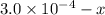 x
x![[FeSCN^{2+}]=1.74\times 10^{-4}M=x](/tpl/images/0528/9422/2c1de.png)
![[Fe^{2+}]\text{ ions}=(1.4\times 10^{-2}-x)=(1.4-0.0174)\times 10^{-3}=1.383\times 10^{-2}M](/tpl/images/0528/9422/f1fb2.png)
![[SCN^{-}]\text{ ions}=(3.0\times 10^{-4}-x)=(3.0-1.74)\times 10^{-4}=1.26\times 10^{-4}M](/tpl/images/0528/9422/6910c.png)
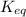 for above equation follows:
for above equation follows:![K_{eq}=\frac{[FeSCN^{2+}]}{[Fe^{3+}][SCN^-]}](/tpl/images/0528/9422/63e7c.png)