
Chemistry, 29.02.2020 01:51, heyysiirr3064
The first ionization potential of the elements B, C, and N (atomic numbers 5, 6, and 7) steadily increases, but that of O is less than that of N. The best interpretation of the lowervalue for O is that:1. the ionization potential of N is a maximum and the values decrease steadily for the elements O, F, and Ne.2. there is more shielding of the nuclear charge in O than in B, C, or N.3. the electron removed from O is farther from the nucleus and therefore less tightly bound than that in N.4. the electron removed from O corresponds to a different value of the quantum number L than that of the electron removed from B, C, or N.5. the half-filled set of p orbitals in N makes it more difficult to remove an electron from N than from O.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, ebigham5117
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 16:50, lilblackbird4
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 18:00, jeepjose58
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1
Do you know the correct answer?
The first ionization potential of the elements B, C, and N (atomic numbers 5, 6, and 7) steadily inc...
Questions in other subjects:

Physics, 05.02.2021 17:40





Spanish, 05.02.2021 17:40


Mathematics, 05.02.2021 17:40


English, 05.02.2021 17:40


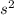 2
2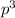 (half-filled orbital), Which allows it additional stability.
(half-filled orbital), Which allows it additional stability.



