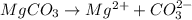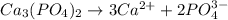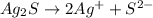Chemistry, 29.02.2020 01:24, leaving2020asap
Write the ion-product expression at equilibrium for each compound: How to enter the correct e. g. silver sulfate (Ag2SO4) enter this: {Ag+}2{SO42-} don't enter spaces, use square brackets [] not {} or () for concentration and don't worry about subscripting or superscripting [ (1) magnesium carbonate (2) calcium phosphate (3) silver sulfide

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 03:50, mobslayer88
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2

Chemistry, 23.06.2019 09:00, aaronroberson4940
Weight is a measure of: inertia force matter mass
Answers: 1

Chemistry, 23.06.2019 10:30, dreamxette3119
Fill in the blanks for the following statements: the rms speed of the molecules in a sample of h2 gas at 300 k will be times larger than the rms speed of o2 molecules at the same temperature, and the ratio µrms (h2) / µrms (o2) with increasing temperature. a not enough information is given to answer this question b sixteen, will not change c four, will not change d four, will increase e sixteen, will decrease
Answers: 2
Do you know the correct answer?
Write the ion-product expression at equilibrium for each compound: How to enter the correct e. g. s...
Questions in other subjects:


Mathematics, 22.01.2021 20:00




Social Studies, 22.01.2021 20:00




History, 22.01.2021 20:00


![Q_{MgCO_3}=[Mg^{2+}][CO_3^{2-}]](/tpl/images/0528/8473/505d7.png)
![Q_{Ca_3(PO_4)_2}=[Ca^{2+}^3][PO_4^{3-}^2]](/tpl/images/0528/8473/bdee3.png)
![Q_{Ag_2S}=[Ag^{+}^2][S^{2-}]](/tpl/images/0528/8473/94d2a.png)