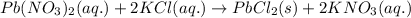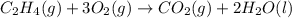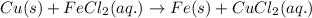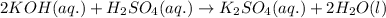Classify each of these reactions. Pb ( NO 3 ) 2 ( aq ) + 2 KCl ( aq ) ⟶ PbCl 2 ( s ) + 2 KNO 3 ( aq ) C 2 H 4 ( g ) + 3 O 2 ( g ) ⟶ 2 CO 2 ( g ) + 2 H 2 O ( l ) Cu ( s ) + FeCl 2 ( aq ) ⟶ Fe ( s ) + CuCl 2 ( aq ) 2 KOH ( aq ) + H 2 SO 4 ( aq ) ⟶ K 2 SO 4 ( aq ) + 2 H 2 O ( l )

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, RidhaH
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural. question 2 reflects a moral or social value. question 3 refers to something that can be measured. question 4 reflects a question that can’t be observed.
Answers: 1


Chemistry, 23.06.2019 00:00, jasmin5285
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1

Chemistry, 23.06.2019 05:50, starfox5454
Which of the following isotopes has the same number of neutrons as phosphorus-31?
Answers: 1
Do you know the correct answer?
Classify each of these reactions. Pb ( NO 3 ) 2 ( aq ) + 2 KCl ( aq ) ⟶ PbCl 2 ( s ) + 2 KNO 3 ( aq...
Questions in other subjects:

History, 25.01.2022 02:10


Mathematics, 25.01.2022 02:10

English, 25.01.2022 02:10


History, 25.01.2022 02:10

Mathematics, 25.01.2022 02:10



Mathematics, 25.01.2022 02:10