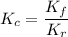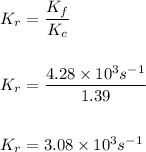Chemistry, 29.02.2020 01:24, thomaswillmsn7496
For a different reaction, Kc = 1.39, kf=12.6s−1 , and kr= 9.08 s−1 . Adding a catalyst increases the forward rate constant to 4.28×103 s−1 . What is the new value of the reverse reaction constant, kr, after adding catalyst?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:20, ayoismeisalex
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 19:00, QuestionsAnsweredNow
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3

Chemistry, 22.06.2019 20:00, SpiritedAway7087
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Do you know the correct answer?
For a different reaction, Kc = 1.39, kf=12.6s−1 , and kr= 9.08 s−1 . Adding a catalyst increases the...
Questions in other subjects:

Mathematics, 19.07.2019 03:00

Mathematics, 19.07.2019 03:00




Mathematics, 19.07.2019 03:00

Mathematics, 19.07.2019 03:00


Mathematics, 19.07.2019 03:00

Biology, 19.07.2019 03:00