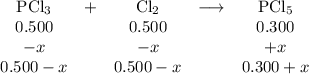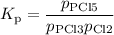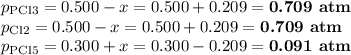Chemistry, 29.02.2020 00:17, Dogtes9667
For the exothermic reaction
PCl3(g)+Cl2(g)?PCl5(g)
Kp = 0.180 at a certain temperature.
A flask is charged with 0.500 atm PCl3 , 0.500 atm Cl2, and 0.300atm PCl5 at this temperature.
What are the equilibrium partial pressures of PCl3 , Cl2, and PCl5, respectively?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:50, hadwell34
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2



Chemistry, 22.06.2019 22:30, eduardoguizar8787
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
Do you know the correct answer?
For the exothermic reaction
PCl3(g)+Cl2(g)?PCl5(g)
Kp = 0.180 at a certain temperature.<...
PCl3(g)+Cl2(g)?PCl5(g)
Kp = 0.180 at a certain temperature.<...
Questions in other subjects:

Mathematics, 23.08.2019 10:00




Biology, 23.08.2019 10:00

Chemistry, 23.08.2019 10:00

Geography, 23.08.2019 10:00

Arts, 23.08.2019 10:00


Chemistry, 23.08.2019 10:00