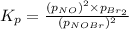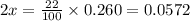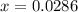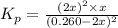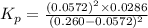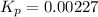Chemistry, 28.02.2020 21:57, alexismurcia550
Nitrosyl bromide decomposes according to the chemical equation below. 2NOBr(g) ↔ 2NO(g) + Br2(g) When 0.260 atm of NOBr is sealed in a flask and allowed to reach equilibrium, 22% of the NOBr decomposes. What is the equilibrium constant, Kp, for the reaction?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, angelinadhar
What are the percent by mass of copper in penny lab
Answers: 3

Chemistry, 22.06.2019 04:00, nothingworksoutforme
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1


Chemistry, 22.06.2019 13:00, monkeyrose1999
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3
Do you know the correct answer?
Nitrosyl bromide decomposes according to the chemical equation below. 2NOBr(g) ↔ 2NO(g) + Br2(g) Whe...
Questions in other subjects:

Spanish, 11.11.2019 19:31

SAT, 11.11.2019 19:31

History, 11.11.2019 19:31

History, 11.11.2019 19:31




Mathematics, 11.11.2019 19:31

History, 11.11.2019 19:31

Mathematics, 11.11.2019 19:31


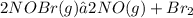 "
" 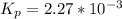
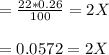
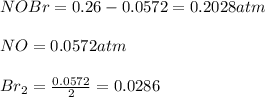
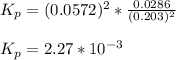
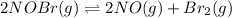
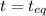 0.260-2x 2x x
0.260-2x 2x x
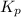 for the given reaction follows:
for the given reaction follows: