
Chemistry, 28.02.2020 20:06, michellen2020
The first two steps in the industrial synthesis of nitric acid produce nitrogen dioxide from ammonia: 2N0(g) +02(g) 2NO2 (g) The net reaction is: 4NH, (g) [email protected])= 4 Write an equation that gives the overall equilibrium constant K in terms of the equilibrium constants K1 and K2. If you need to include any physical constants, be sure you use their standard symbols, which you'll find in the ALEKS Calculator. K=

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 08:40, alexisbcatlett14
Which statement can best be concluded from the ideal gas law?
Answers: 2
Do you know the correct answer?
The first two steps in the industrial synthesis of nitric acid produce nitrogen dioxide from ammonia...
Questions in other subjects:



Social Studies, 04.08.2019 03:20

Biology, 04.08.2019 03:20

Social Studies, 04.08.2019 03:20

History, 04.08.2019 03:20


History, 04.08.2019 03:20



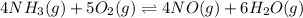
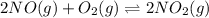
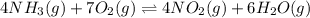
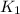 and
and 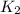 . If you need to include any physical constants, be sure you use their standard symbols
. If you need to include any physical constants, be sure you use their standard symbols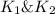 :
: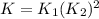
![K_1=\frac{[NO]^4[H_2O]^6}{[NH_3]^4[O_2]^5}](/tpl/images/0528/3548/6d44f.png)
![K_2=\frac{[NO_2]^2}{[NO]^2[O_2]}](/tpl/images/0528/3548/04922.png)
![K=\frac{[NO_2]^4[H_2O]^6}{[NH_3]^4[O_2]^7}](/tpl/images/0528/3548/d6ea1.png)
![[NO]^4](/tpl/images/0528/3548/e5e10.png) ;
;![K=\frac{[NO_2]^4[H_2O]^6}{[NH_3]^4[O_2]^7}\times \frac{[NO]^4}{[NO]^4}](/tpl/images/0528/3548/cc872.png)
![K=\frac{[NO]^4[H_2O]^6}{[NH_3]^4[O_2]^7}\times \frac{[NO_2]^4}{[NO]^4}](/tpl/images/0528/3548/b9d44.png)
![K=K_1\times \frac{[NO_2]^4}{[O_2]^2[NO]^4}](/tpl/images/0528/3548/1f7b8.png)
![K=K_1\times (\frac{[NO_2]^2}{[O_2]^1[NO]^2})^2](/tpl/images/0528/3548/d8543.png)





