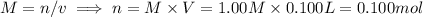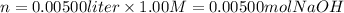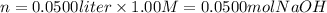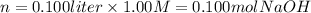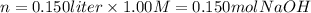Chemistry, 28.02.2020 19:46, robert7248
A) A volume of 100 mL of 1.00 M HCl solution is titrated with 1.00 M NaOH solution. You added the following quantities of 1.00 M NaOH to the reaction flask. Classify the following conditions after the equivalence point. Write down the items to their respective table.
i) 5.00 mL of 1.00 M NaOH
ii) 50.0 mL of 1.00 M NaOH
iii) 100 mL of 1.00 M NaOH
iv) 150 mL of 1.00 M NaOH
v) 200 mL of 1.00 M NaOH
Before equivalence point At equivalence point After equivalence point

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, aylengarcia090
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 12:30, johnsont8377
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1


Chemistry, 22.06.2019 20:00, SpiritedAway7087
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3
Do you know the correct answer?
A) A volume of 100 mL of 1.00 M HCl solution is titrated with 1.00 M NaOH solution. You added the fo...
Questions in other subjects:


Social Studies, 09.05.2021 02:30






Mathematics, 09.05.2021 02:30

Advanced Placement (AP), 09.05.2021 02:30