
Chemistry, 28.02.2020 19:25, kayleegeise
The equilibrium constant for the reaction below, at a given temperature is 45.6. If the equilibrium concentrations of F2 and BrF3 are 1.24 x 10-1 M and 1.99 x 10-1 M respectively, calculate the equilibrium concentration of Br2. (4)

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, emfranco1
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 17:00, princessakosua2
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1
Do you know the correct answer?
The equilibrium constant for the reaction below, at a given temperature is 45.6. If the equilibrium...
Questions in other subjects:

Mathematics, 08.12.2020 19:00


Chemistry, 08.12.2020 19:00

Mathematics, 08.12.2020 19:00


Mathematics, 08.12.2020 19:00



English, 08.12.2020 19:00

English, 08.12.2020 19:00


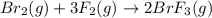
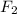 at equilibrium =
at equilibrium = 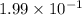
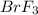 at equilibrium =
at equilibrium = 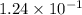
![K_c=\frac{[BrF_3]^2}{[Br_2][F_2]^3}](/tpl/images/0528/1843/a1098.png)
![45.6=\frac{(1.24\times 10^{-1})^2}{[Br_2]\times (1.99\times 10^{-1})^3}](/tpl/images/0528/1843/8cd8d.png)
![[Br_2]=0.0428M](/tpl/images/0528/1843/2a0dc.png)




