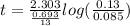Chemistry, 28.02.2020 19:21, semajac11135
The half-life of a first-order reaction is 13 min. If the initial concentration of reactant is 0.13 M, it takes min for it to decrease to 0.085 M. The half-life of a first-order reaction is 13 min. If the initial concentration of reactant is 0.13 M, it takes min for it to decrease to 0.085 M. 10. 11 7.0 12 8.0

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, kaliloabousjbf
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3



Chemistry, 22.06.2019 19:50, mikaylaaaaa
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
Do you know the correct answer?
The half-life of a first-order reaction is 13 min. If the initial concentration of reactant is 0.13...
Questions in other subjects:

English, 24.03.2020 21:08


Mathematics, 24.03.2020 21:08


Mathematics, 24.03.2020 21:08





Mathematics, 24.03.2020 21:09


 λN
λN t time t = 0.
t time t = 0.

 = 13 min
= 13 min
![\textrm{rate of reaction}=-\frac{d[A]}{dt} =k[A]](/tpl/images/0528/1630/bb97d.png)
![k=\frac{2.303}{t} log\frac{[A_0]}{[A]}](/tpl/images/0528/1630/263ab.png)

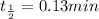


![\Rightarrow t=\frac{2.303}{k} log\frac{[A_0]}{[A]}](/tpl/images/0528/1630/3dc05.png)
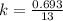 , [A₀] = 0.13 m and [ A] = 0.085 M
, [A₀] = 0.13 m and [ A] = 0.085 M